What are Fluorescent Minerals & Rocks? | Ultraviolet Tools LLC
Welcome to the fascinating world of fluorescence.
The invisible ultraviolet radiation can change a dull piece of stone into an explosion of light in all imaginable colors of the rainbow. How is this seemingly magical process possible? I will try to explain how this can happen, talk about the everyday applications of fluorescence, the hazards, the lamps and filters and of course you can view some minerals in the gallery.
About 1850, a scientist, Sir George Stokes, was amazed that Fluorite was green in the shade and strong blue in direct sunlight. This ability to change color under a different light source, he named "fluorescence". Fluorescence is the light-emission by certain substances when they are activated by ultraviolet energy (light). The emission disappears when the activation is removed.
What is light?
To fully understand fluorescence it is necessary that we first see what light is. Light is a type of electromagnetic radiation emitted by the sun and other light sources. That electromagnetic radiation comes in various wavelengths. The wavelength is measured in nanometers (1 nm = 1/millionth of a millimeter).
The figure below is a schedule of the whole electromagnetic spectrum. Look at the types of radiation from right to left.
Radio waves are similar to light, only their wavelength is much larger. Then the microwave, which are commonly used in radar and example in the microwave to heat food from the inside. Then you get infrared radiation, which is used in the remote control.. Infrared is abbreviated as IR, people feel it as heat.
Then comes the visible light, as you can see it is just a small part of the whole spectrum of electromagnetic radiation. Light is made up of different colors. All colors have different wavelengths. Wavelength is defined as the distance between two peaks of waves, as you also see when you throw a rock in the water. The color of an object is determined by the frequency (wavelength) of the electromagnetic radiation that reflects back. A black object absorbs all wavelengths of visible light. A red object absorbs all wavelengths except the radiation of that frequency that causes the red light.
Then comes the ultraviolet light, or also called: UV light. We can neither see of feel this light. It is the ultraviolet component of sunlight, for example, that causes our skin to sunburn and tan.
The X-rays, which are widely used in medical science, and finally, the gamma rays, these rays are harmful to all living beings, they are used in nuclear power plants (uranium) for example, gamma rays have the shortest wavelength, but has the highest energy value.
Ultraviolet Light
Ultraviolet radiation is in the schedule before the visible light. The energy value is higher! The wavelengths are from 100 nm to 400 nm. We can divide ultraviolet light into four areas, as you can see in the figure above. The long wave radiation are in the range of 315-400 nm. (Also called UV-A). The term "black light" is a well-known in dance clubs and is widely used, because of the glowing (fluorescence) effect. It is often used in clothes, many paints, and to give paper more whiteness. Many minerals in are fluoresce in this light, the best known is fluorite. The area in the medium range (280-315 nm). Some minerals, such as scapolites, respond to the medium wave radiation. The radiation around 300-310 nm. (UVB) causes a photochemical reaction in the skin, what we experienced as sunburn. The production of vitamin D is also affected by this radiation.
The short wave radiation is the area of 200 to 280 nm. (UV-C) but we will not normally reach. This radiation is blocked by the atmosphere. It is this radiation (from below 300 nm.) Which has a bactericidal effect, making it much used in the food industry. Vacuum ultraviolet light is the last area, and not for use in fluorescence of minerals.
Some Applications of Fluorescence
Fluorescence is widely used.
- It is often used in stamps and postmarks so they can be sorted automatically. Recognizable are the almost invisible stripes stamped on the envelope.
- Printing paper is often bleached, so the normal light (which includes long wave UV), makes the paper appear whiter!
- Banknotes haves UV markings to avoid falsification.
- Fluorescent ink is used in clothing, advertising, art, music videos, theater shows and advertising lights.
- In criminology are fingerprints detected with fluorescent powder.

- In the uranium mining industry the mineral was often easier to find with a UV lamp or with a Geiger counter.
- In ophthalmology, the wounded or infected eyes are treated with fluorescein, after illuminating the eye with a UV lamp, the damage is clearly visible.
- In laboratories are numerous applications for preparations visible.
- As mentioned earlier it is frequently used in the food industry for the bactericidal activity, as water is also often treated with UV lamps in pond pumps.
Mineral Fluoresce
To understand how minerals fluoresce, we look at how minerals are structured and how they react to ultraviolet light. In the nucleus, protons and neutrons are surrounded by electrons, circling round the nucleus. The electrons absorb the energy of ultraviolet light (ionisation).
Fluorescence in Schedule
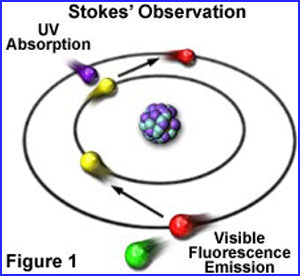
An electron absorbs the UV energy. The electron receive an energy boost (ionisation) and moves temporarily to a higher shell, but the electron cannot hold its position, and falls back to its old place.
During the whole process a part of the energy is lost. The lower energy now released, is transferred into light, in a visible range, of a lower wavelength. The broadcasting color is depending of that frequency.
The UV Lamps
The two main types of UV lamps used for mineral fluorescence are the longwave and the shortwave.
The normal black-light (long wave) tube is coated on the inside with a phosphor layer. In the tube vacuum is created and Argon gas is added with a little mercury. When the lamp is turned on, the current between the cathodes vaporizes the mercury and causes the mercury vapor to emit copious quantities of ultraviolet light. This light is absorbed by the phosphor coating which lines the tube, and is re-emitted as long wave black light. This is also used in the normal fluorescent lights which light up our offices, the difference is that the phosphor coating then emitted visible light.
The short wave radiation is invisible and does not penetrate most glass and plastics. The short wave UV lamps are therefore not glass but quartz or high silicate glass. Nearly 90% of the energy of the short wave UV light is emitted as light of 254 nm., 3.8% as heat and 7% other visible light. This little bit of visible light, ruins a lot of the effect of florescence. That's why the lamps are fitted with a filter. The filter is the most expensive part of the lamp (about 60%).
What are the causes of fluorescence?
Nearly all minerals with a metallic or submetallic luster show no visible fluorescence under ultraviolet light. The purer the mineral, the less chance they are fluorescent. Nearly all fluorescent minerals are poor conductors (electrical insulators).
Impurities (contamination of other substances) Uranyl ion (UO2) 2 + in Opal and chalcedony Manganese (Mn2 +) ion is one of the main causes of fluorescence. Chromium (Cr 3 +) ion in emerald or disulphide (S2) in sodalite
Some minerals fluoresces because they have a part of the molecule which is always fluoresce, this is called Intrinsic factor (internal factors by the presence of certain elements in the molecules). Uranyl ion (UO2) 2 + lead or mercury, some titanium ions (WO4) 2 - (tungstate ion) or (MoO4) 2 - Molhybdate
Crystal defects (see illustration)
Defects in crystal lattices
crystal defects
Other forms of light emitting materials
Phosphorescence: a mineral will emit light after the UV light source is off. (Afterglow)
Thermoluminescence: light emitted by a heated substance.
Bioluminescence: light emitted by bacteria/fungi during the decomposition process or slow oxidation of life forms (fireflies)
Cryoluminescentie: when a substance become fluorescent at very low temperatures.
Tenebrescentie: The mineral has a different color in daylight after exposure to UV light. (Sodalite)
Triboluminescentie: the light is emitted when mineral is scratched or struck. (Tremolite)Ultraviolet light and the damage to humans
As mentioned earlier, UV light (short wave), causes sunburn. This can also happen to your eyes, when you look directly in the UV-lamp . If you do look into the lighted shortwave lamp for a longer time you will get sunburned eyes. The eyes get weepy and feel like they have sand in them when you blink. This will disappear in a few days and can be supported with analgesic eye drops. Glasses or protected goggles will protect you. Never look into any UV light source, it damage your eyes.
- In the uranium mining industry the mineral was often easier to find with a UV lamp or with a Geiger counter.
